Cell Analyzers
Calcium Flux
In their resting state, eukaryotic cells maintain an internal calcium ion concentration that is far below that of the extracellular environment. Ionized calcium plays an important role as a mediator of transmembrane signal transduction, and elevations in intracellular ionized calcium concentration ([Ca2+]i) regulate diverse cellular processes.
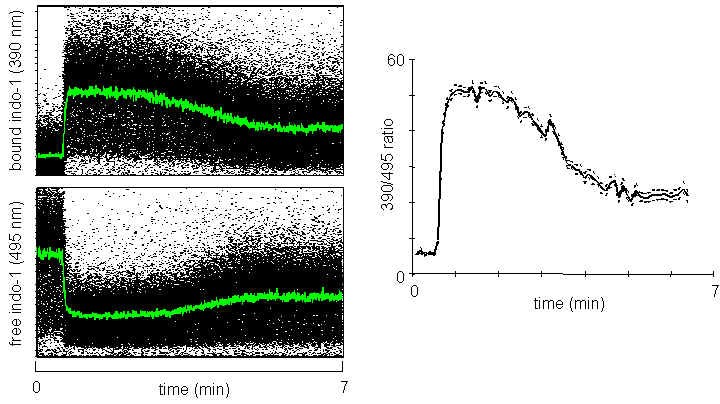
The principle of flow cytometric Ca2+ flux measurement is based on changes in fluorescence intensity or emission wavelength of a fluorophore following the chelation of calcium ions. This is commonly reported as a plot of fluorescence intensity against time. Furthermore, a ratiometric method is employed to minimize the effect of cell-to-cell variation on the final analysis (see figure). Indo-1 is the ratiometric calcium indicator dye most commonly used in flow cytometry because of its shift in emission frequency when excited at a single wavelength. Indo-1 is excited by a UV laser line available on our cell analyzers with custom dedicated filter sets. Other available dyes include Fluo-3 or Fluo-4 and Fura Red – fluorescein-based, calcium-sensitive probes that are both excited at 488 nm. A ratiometric analysis with visible excitation is possible using two dyes simultaneously: Fluo-4 fluoresces in the green region with increasing intensity when bound to calcium, while Fura Red exhibits the inverse behavior, fluorescing most intensely in the red region when not bound to calcium.
Ionomycin at 1 μM is added to the cell population following the start of data acquisition. The dot plots on the left show the fluorescence emission at 420 nm (bound Indo-1) and 510 nm (free Indo-1). Both wavelengths are collected on a linear scale. The plot on the right shows the ratio between 420 and 510 nm.
Proper controls are necessary to determine Ca2+ indices. Ionomycin is an ionophoric antibiotic that functions as a mobile carrier of calcium ions, transporting them through the cell membrane and releasing them into the cytoplasm. The compound is employed as a positive control to determine the maximum loading rate of intracellular calcium. EDTA and EGTA are chelators of cations, and when present in solutions, they cause cations such as Ca2+ to be unavailable for cellular functions. Use ionophores as positive controls and calcium chelators as negative (Ca2+ low) controls in calcium flux protocols.
Calcium Measurements with Indo-1
The acetyloxymethyl ester form of Indo-1 AM is cell-permeant, but intracellular esterases cleave the acetyl groups, rendering Indo-1 impermeant. Indo-1 is excited by UV light and fluoresces at different wavelengths depending on whether it is bound to calcium (~420 nm) or free (~510 nm). There is a 6-fold increase in signal when cells change from basal levels of Ca2+ to saturating levels. The ratio of these two wavelengths can indicate changes in intracellular calcium concentration. The optimal concentration of Indo-1 varies with cell type, ranging from 1 to 10 μM. This typically requires empirical determination, but concentrations at the low end of the range tend to yield more sensitive measurements. Cells loaded with Indo-1 are analyzed relative to a time parameter, and the change in fluorescence ratio over time can be related to changes in activation or stimulation by some agonist that elicits a calcium flux.
Calcium measurements with Indo-1 can be acquired on our cell analyzers with specific filters. Additionally, we have options to maintain the sample temperature at 37°C to more accurately correspond to in vivo conditions. For further information or assistance with Indo-1 measurements, please contact us.