Cell Analyzers
Cell Signaling
Intracellular kinase signaling cascades are driven by the phosphorylation of signal transducers and activators of transcription. Exposing cells to signaling molecules, such as cytokines and growth factors, will affect these proteins’ phosphorylation levels and activation state. The phosphorylation of signal transducer proteins is reversible and transient, making it possible to use them as dynamic indicators of intra-cellular activity status.
The ability to measure the phosphorylation state of specific proteins has been achieved through the development of phospho-specific antibodies targeting particular phospho-epitopes. These phospho-specific antibodies were first used in Western blotting. Applying this technique to a heterogeneous lymphocyte population can create the impression of a uniform cellular response to a stimulus. However, using phospho-specific antibodies in flow cytometry offers a very appealing advantage: it can determine which signaling cascades are activated in response to a stimulus and define the signaling kinetics—all at the single-cell level. The flow-cytometric single-cell approach allows for the simultaneous analysis of multiple epitopes in complex heterogeneous populations, such as peripheral blood cells or murine splenocytes. Additionally, monitoring cellular signaling with flow cytometry has a large dynamic range of data collection (up to 10,000-fold) and a rapid protocol that takes about 2 hours, compared to the 1-2 days needed for Western blots.
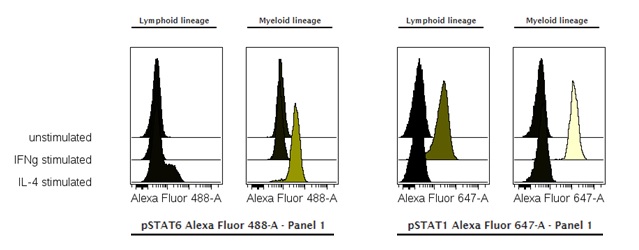
Jurkat and U397 cells were stimulated with IFN-γ and Il-4 and stained with phospho-Stat1 and phospho-Stat6.